Introduction to Thermodynamics
Thermodynamics, a fundamental branch of physics and engineering, deals with the study of energy and its transformations. It provides essential principles governing the behavior of energy and matter in various systems. Understanding thermodynamics is crucial for advancements in science, technology, and industry.
First Law of Thermodynamics
At the heart of thermodynamics lies the First Law, which states that energy cannot be created or destroyed; it can only be transformed from one form to another. This principle, also known as the law of conservation of energy, underscores the fundamental nature of energy in the universe. Whether it’s mechanical work, heat transfer, or chemical reactions, the total energy within a closed system remains constant.
Second Law of Thermodynamics
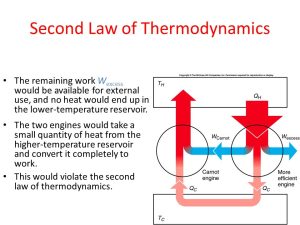
In contrast to the First Law, the Second Law introduces the concept of entropy, a measure of disorder or randomness in a system. It states that in any energy transformation process, the total entropy of an isolated system tends to increase over time. This law highlights the irreversibility of certain processes and the tendency of systems to move towards equilibrium.
Constraints on Energy Transformation
The Second Law imposes constraints on the efficiency of energy conversion processes. One of the notable concepts derived from the Second Law is the Carnot efficiency, which represents the maximum efficiency achievable for a heat engine operating between two temperature reservoirs. This theoretical limit underscores the inherent limitations in converting heat into useful work.
Applications of Thermodynamics
Thermodynamics finds widespread applications in various fields, including engineering, chemistry, and environmental science. It underpins the design and operation of heat engines, refrigeration systems, and chemical processes. By understanding thermodynamic principles, engineers can optimize energy usage, improve efficiency, and minimize environmental impact.
Challenges and Controversies
Despite its wide acceptance, the Second Law of Thermodynamics has sparked debates and controversies over the years. Alternative interpretations and theories have emerged, challenging conventional views on entropy and energy transformation. These debates fuel ongoing research and exploration into the fundamental principles of thermodynamics.
Conclusion
Thermodynamics and the Second Law play a vital role in shaping our understanding of energy transformation and the behavior of physical systems. By grasping these principles, scientists and engineers can unlock new possibilities for innovation and sustainable development.
FAQs
Is the Second Law of Thermodynamics absolute?
The Second Law is a fundamental principle based on empirical observations, but its application may vary in certain contexts.
What is the significance of entropy in thermodynamics ?
Entropy quantifies the dispersal of energy in a system and provides insights into the directionality of processes.
How does thermodynamics impact everyday life?
Thermodynamic principles influence various aspects of daily life, from household appliances to industrial processes.
Can we overcome the limitations imposed by the Second Law?
While the Second Law sets theoretical constraints, advancements in technology may enable us to improve efficiency and mitigate energy losses.
What are some emerging areas of research in thermodynamics?
Current research focuses on nanoscale thermodynamics, quantum thermodynamics, and the thermodynamics of complex systems.








